- TOP
- Company Information
- Corporate Profile
Corporate Profile
- Corporate name
- YUKI GOSEI KOGYO CO., LTD.
- Offices
- Head Office, Business DivisionⅡ, Tokyo Research Laboratory, Joban Factory, Düsseldorf Office
- Business Fields
- ・Pharmaceuticals
・Food additives
・Industrial chemicals
- Capital
- 3,471 million yen (total number of issued shares: 21,974,000 shares)
Listed on the Tokyo Stock Exchange, Standard Market
- Executives
(as of June 21, 2024) -
President & CEO
Business Sector
Seiichiro MatsumotoDirector & Managing Executive Officer, Research & Development Sector, Manufacturing Sector
Masahiro KusanoDirector & Senior Executive Officer, Business Administration Sector
Tatsuya KomatsubaraDirector, Audit & Supervisory Committee Member
Naotake SutoDirector, Audit & Supervisory Committee Member
Keisuke YamadaDirector, Audit & Supervisory Committee Member
Norito OhoriSenior Executive Officer, General Manager, Business DivisionⅠ & Business Management Division
Masao MatsukawaExecutive Officer, General Manager, General Affairs & Human Resources Division
Hiromi IshikawaExecutive Officer, General Manager, Joban Factory
Makoto KitoExecutive Officer, General Manager, Quality Assurance Division
Hiroshi Nakamura
- Number of employees
(as of March 31, 2024) - 290 (excluding loan, part-time and temporary employees)
- Major banks
- MUFG Bank, Ltd., Joyo Bank, Mitsubishi UFJ Trust and Banking Corporation, Mizuho Bank, Ltd.
- Affiliated company
- Yuki Techno Service Co., Ltd.
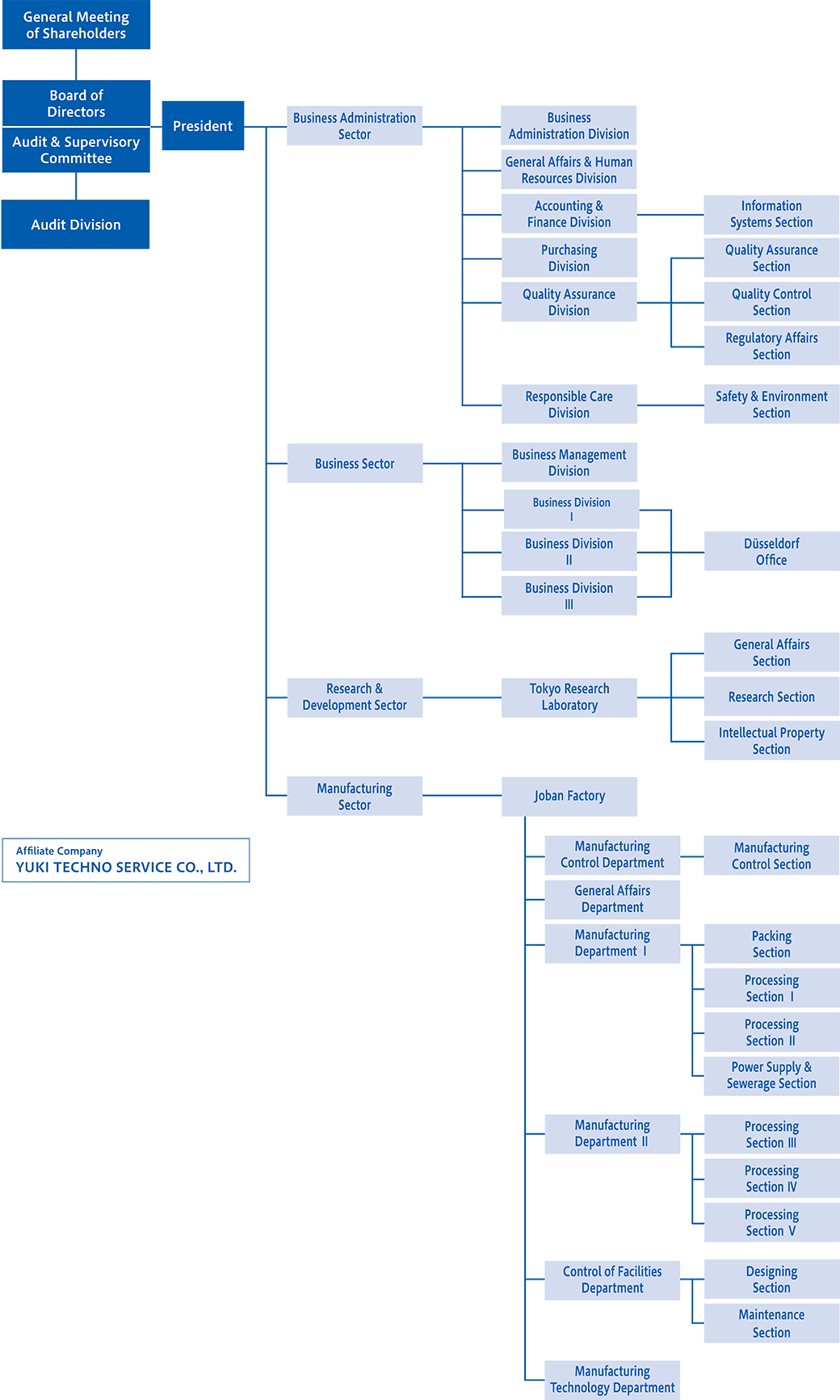
Organizational Chart
History
1947
Yuki Gosei Kogyo Kabushikigaisha (Yuki Gosei Kogyo Co., Ltd.) was established to produce flavors for tobacco
-
1948
Acquired Maeno Plant (later renamed“Maeno Office”) -
1952
Started manufacture of glycine, isoniazid and nicotinic acid amide -
1958
Started manufacture of beta-alanine and pinkmine -
1960
Started manufacture of 2-vinylpyridine -
1961
Started manufacture of glycine and beta-alanine using a new manufacturing method -
1962
The company’s name in Japanese changed from“Yuki Gosei Kogyo Kabushikigaisha”to“Yuki Gosei Yakuhin Kogyo Kabushikigaisha”
The Head Office moved to Takara-cho, Chuo-ku
Listed on the Second Section of the Tokyo Stock Exchange -
1963
Started manufacture of triacetin -
1964
Started operation of Joban Plant
Started manufacture of flavors for Peace cigarettes -
1965
Started manufacture of protamine sulfate and flavors for mf cigarettes -
1966
Started manufacture of leucic acid and flavors for Hi-lite cigarettes -
1967
Started manufacture of nicotinic acid amide using a new manufacturing method
Started manufacture of 3-cyanopyridine -
1968
Started manufacture of 8-hydroxyquinoline and flavors for Seven Stars cigarettes -
1969
Started manufacture of amino acid sugar (Yukisu) -
1970
Started manufacture of flavors for Cherry cigarettes -
1971
Started manufacture of glycine at new facilities -
1972
Started manufacture of nicotinic acid amide at new facilities
Tokyo Laboratory completed -
1973
Started manufacture of iminodiacetic acid -
1975
Started manufacture of intermediates for agricultural chemicals and picolinic acid -
1976
Started manufacture of CP essence -
1977
Started manufacture of 3-aminopyridine -
1978
Started manufacture of DNA
Activated sludge treatment facilities completed at Joban Plant -
1982
Test plant facilities newly installed at Tokyo Laboratory -
1983
Facilities for manufacturing flavors and materials for tobacco newly installed at Joban Plant -
1984
Multi-purpose manufacturing facilities newly installed at Joban Plant -
1985
Started manufacture of silicone-related products -
1986
Started manufacture of 4-aminopyridine and isonicotinic acid -
1990
Drug intermediate-purification facilities newly installed at Joban Plant
Capital increased to 2,550 million yen -
1991
The Head Office moved to Hirakawa-cho, Chiyoda-ku
Manufacturing facilities for silicon and grignard reagents newly installed at Joban Plant -
1992
Multi-purpose, small-amount manufacturing facilities newly installed at Joban Plant -
1995
The Head Office moved to the current location
A laboratory (Technological Development Center) newly established at Joban Plant
DNA manufacturing facilities newly installed at Joban Plant -
1996
The 2-vinylpyridine manufacturing facilities moved from Tokyo Laboratory to Joban Plant
Joban Plant certified for ISO 9002.
Manufacturing facilities dedicated to silicon- and grignard-related products newly installed at Joban Plant
Capital increased to 3,471 million yen
Started manufacture of trifluorothymidine -
1998
The Head Office, Osaka Sales Office, Joban Plant and Tokyo Laboratory certified for ISO 9001
Investigational drug testing research facilities newly installed at Joban Plant -
1999
Certified for ISO 14001 -
2001
Started manufacture of Fudosteine “YUKI”
Yuki Engineering Co., Ltd., an affiliated company, renamed “Yuki Techno Service Co., Ltd”
Pharmaceutical manufacturing facilities newly installed at Joban Plant -
2003
Selected as loanable stock
Quality Control Building newly completed at Joban Plant. -
2004
Listed on the First Section of the Tokyo Stock Exchange market
Started operation of the enterprise resource planning system -
2006
Dusseldorf Office established as an overseas office -
2007
LNG conversion facilities newly established -
2012
Started manufacture of active pharmaceutical ingredients for generics -
2014
Cogeneration system introduced -
2018
APIs and pharmaceutical intermediates manufacturing facilities installed at Joban Plant -
2021
Certified for FSSC 22000 -
2022
Changed to the Standard Market of the Tokyo Stock Exchange